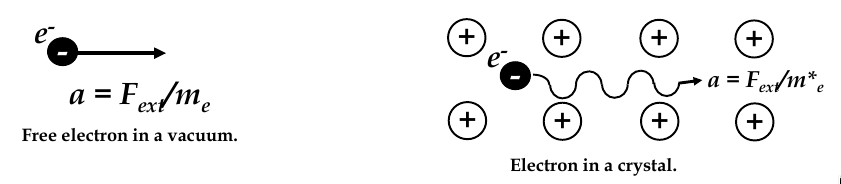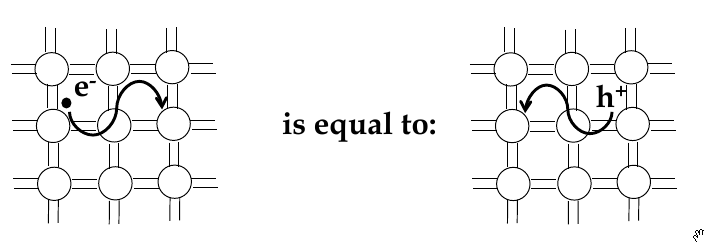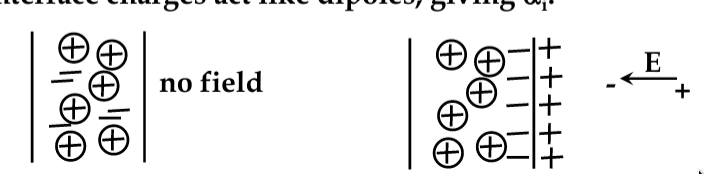ECE 209 Notes
Table of Contents
-
Materials Structure
-
Atomic Structure
-
Band Structure
-
Metals
-
Optics
-
Dielectrics
-
Thermal Properties
-
Magnetic Properties
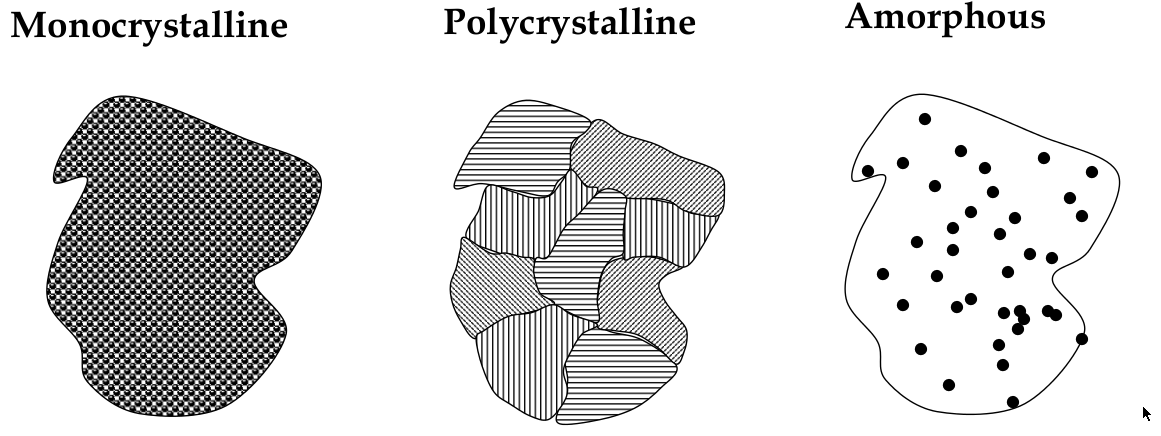
Materials Structures
Crystalline, polycrystalline, and amorphous
-
materials can be classified into the 3 types: crystalline, polycrystalline, and amorphous
Crystalline
-
crystalline solid:
a solid in which atoms bond in a regular pattern to form a periodic array of atoms
-
long range order:
happens in a crystalline solid b/c periodicity;
-
means that each atom is in the same position relative to its relative
-
perfect order yay
Polycrystalline
-
long-range order exists over small distances only
-
has small crystal “grains” that are randomly oriented
Amorphous
-
no long-range order! It’s completely disordered
Crystals in microelectronics
-
different types of crystals should be used for different things in microelectronics
-
Polycrystalline silicon used for:
-
gate material in MOS transistors
-
interconnect lines
-
Amorphous silicon used for:
-
switching transistors for AMLCD displays
-
solar cells
-
crystalline used for all kinds of things
-
attractive because of perfect order, which:
-
simplifies theories
-
repeatable, predictable and uniform properties for material processing
Silicon structure; covalent bonding
-
covalent bonding: shares atoms to make a full valence shell (8 atoms for Si)
-
Si ends up in a tetrahedral shape due to the repulsion interactions
-
silicon has a diamond unit cell
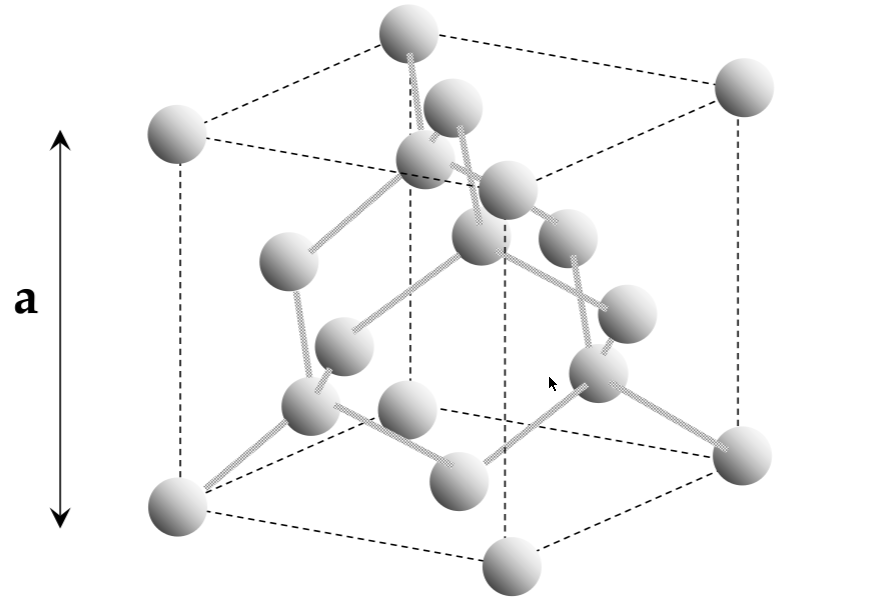
Unit cell
-
lattice:
infinitely repeating array of geometric points in space
-
lattice crystal structure:
a lattice, with atoms on the lattice points
-
unit cell:
smallest repeating structure in the lattice crystal structure
-
lattice constant:
the length of the cubic unit cell - a
-
interatomic distance:
the distance between atoms in a unit cell (not the same as a!!)
Bragg’s Law
-
to measure the lattice constant of an atom, use x-ray diffraction
-
for a wave incident on a plane of atoms, reflective pattern will have bright and dark spots from constructive and destructive interference
-
Bragg’s Law for where bright spots appear:
$$ n\lambda = 2dsin\theta $$
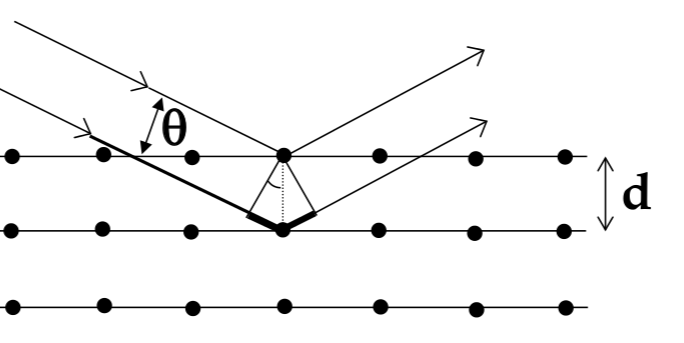
- crystal characteristics (and x-ray diffraction) depend on the direction you are looking
Miller indices
-
since direction matters, we need a way of classifying it
-
miller indices: sets of 3 numbers that are used to identify groups of crystal planes and directions
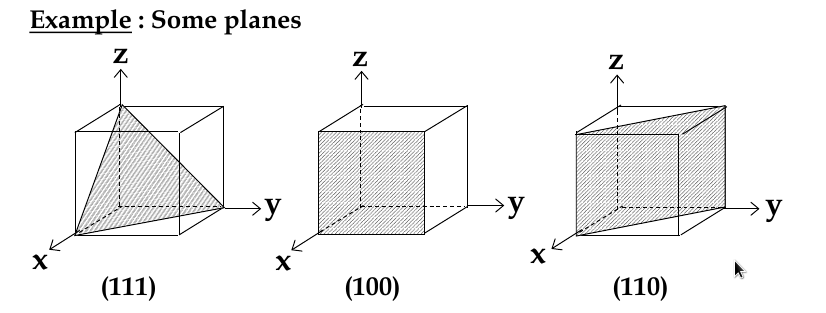
Miller Indices for Planes
-
set up 3 axes along 3 adjacent edges of unit cell
-
choose unit cell length as unit distance along respective axis (a = 1)
-
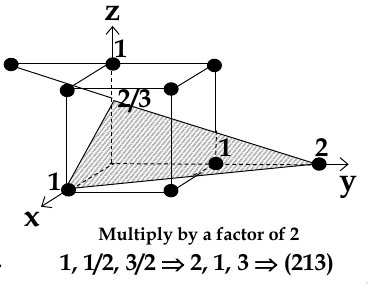
chose a plane that passes through the centre of particular atoms. The plan intersects the axes at distances x1,y1, za (in example below, 1,2, 2/3)
-
take reciprocals of interception co-ordinates, change to set of smallest ints, write as (hkl)
Miller Indices for Directions
-
take a parallel line which passes through the origin
-
not the length of the projections of this line on x,y,z axes
-
change to smallest ints
-
write as [hkl]
Family of Planes and Directions
-
family of planes: {hkl)
-
family of directions:
-
represents all equivalent planes/directions
-
{110} represents all planes (110), (011), (101), etc
Transmission Electron Microscopy
-
TEM samples thinned and illuminated with accelerated electrons
-
electrons are absorbed in the sample depending on thickness and material composition
-
intensity variation of the transmitted electron beam is observed using a viewing screen
Scanning Tunneling Microscopy
-
scans across the surface of sample with a very sharp needle
-
needle kept 1nm from surface, voltage applied between needle and sample
-
current used as feedback signal to determine gap size (can only give information about surface of sample)
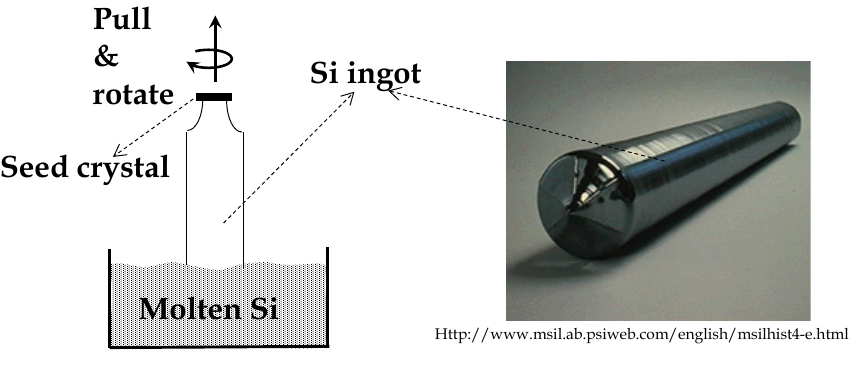
Silicon bulk crystal growth
-
to make ICs, we have to grow perfect crystals on a commercial scale
-
for crystal growth, a saturated solution or a molten liquid is usually used.
-
the material is then grown on a
seed
crystal which acts as a
template
for the new growth
-
for silicon:
-
raw material: silicon dioxide
-
reduction => metallurgical grade polycrystalline Si
-
purification => electronic grade polycrystalline Si
-
melting & growth => crystalline bulk Si
-
during melting and growth, a seed crystal is pulled slowly out of a bath of molten and rotated slowly
-
this is Czochralski (Cz) crystal pulling
-
after growth, ingot is trimmed and sliced into wafers
Epitaxial growth
-
electrical properties of Si are controlled roughly when the basic wafers are produced, but more precise cotrol is needed for reliable devices
-
the top few microns of the wafer are where devices are made
-
this region must be well controlled
-
achieved by growing a even more perfect crystal layer on top of the wafer
-
called the
epitaxial layer
-
during epitaxial growth, the surface of the wafer acts as the template
-
decomposes Si-containing gases in chemical vapor dposition
-
monolayer
: a layer one atom thick
Molecular beam epitaxy
-
a technique for growing thin layers
-
a steam of neutral atoms or molecules are evaporated from a heated cell
-
then incorporated into the growing film onto a heated target
-
is
$$$
$ b/c:
-
only one wafer can be used at a time
-
wafer must be small to get uniform layer
-
very good vacuum required
Epitaxy principles
-
major feature of epitaxy is that the newly deposited film precisely follows the crystalline form of the substrate template
-
adsorption: proces of atoms attaching themselves to the surface
-
adsorption can occur anywhere on the surface with equal probability, so layer is unlikely to be crystalline
-
for crystalline growth, absorbed atoms must be able to find the
minimum energy positions
-
the ad-atoms must be able to move along the surface
-
heat up the substrate during epitaxy to allow movement
-
if not hot enough, the ad-atoms stick where they land and the film is amorphous
Modifying Crystals
-
to make an electronic device, crystals need to be modified and shaped according to the needs of the device
-
examples: introducing impurities, etching/shaping
Ion implantation
-
ionized impurities are accelerated into an electric field and “smashed into” Si surface
-
depth of penetration determined by:
-
accelerating voltage
-
masses of ions and target atoms
-
crystal direction (density of atoms)
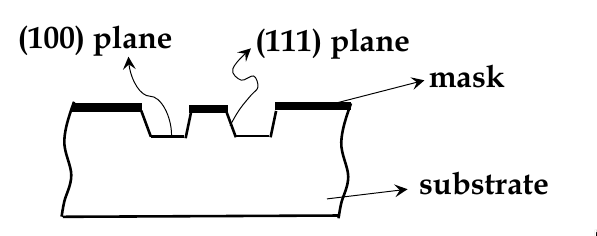
Etching and micromachining
-
sometimes you need to etch crystals to get certain structures
e.g. for making DRAMs, you need to etch deep tranches to make trench-capacitors
-
wet-etching: uses liquid chemicals to remove materials from a wafer
-
isotropic etching: chemicals etch at the same rate in all directions
-
anisotropic etching: chemicals selectively etch one crystal plane more
-
see example below. KOH etches (100) faster than (111) planes
Atomic Structure
Nature of light
-
classic physics: light is an electromagnetic wave w/ perpendicular field Bx and Ey
-
electric field is given by the following equation:
$$ E_y = E_o sin(kx - \omega t) $$
-
where:
-
k = 2π/λ - the wavenumber (λ is the wavelength of light)
-
ω = 2πf - the angular frequency (f is the freq of light)
-
c= ω/k = fλ - speed of light / wave velocity
-
light intensity is given by:
$$ I = \frac{1}{2} c \epsilon_o E_o^2 $$
Experimental Evidence of Light as EM Wave
-
interference and diffraction
-
Young’s double-slit experiment
Photo-emission (photoelectric effect)
-
when a metal electrode is illuminated with light, it emits electron (can create a current with this!)
-
light must possess the energy needed to “free” the electron from the metal (W)
-
any excess energy it possesses will become the kinetic energy of the electron
-
according to classical theory of light, the energy balance should be: E
L
= W + E
K
-
if we reduce E
L
by reducing the intensity of the light, E
K
should also decrease
-
if light intensity is increased -> saturation current increases
-
more electrons emiited
-
same voltage is required to stop the current, thus the kinetic energy of the electrons is the same
-
classical theory of light can’t explain this!
Photons
-
light contains particle of fixed energy called
photons
-
light frequency increases -> energy of light increases
-
E
L
= hf
Wave-Particle Duality
-
light has properties of both a wave and a particle
-
light waves consist of a stream of photon particles, each with energy hf
-
energy carried by the wave consists of
discrete lumps
or
quanta
De Broglie relationship
-
electrons also have a wave-particle duality
-
particle-like properties confirm with cathode ray tube (1897)
-
deBroglie predicted that electrons would have a wavelength:
$$ \lambda = \frac{h}{p} $$
-
where p = mv is the electron momentum
-
confirmed with electron diffraction experiment
Electron Diffraction Experiment
-
voltage accelerates electron, strikes a thin carbon layer, hits the screen
-
produce a glow of light proportional to their number and energy
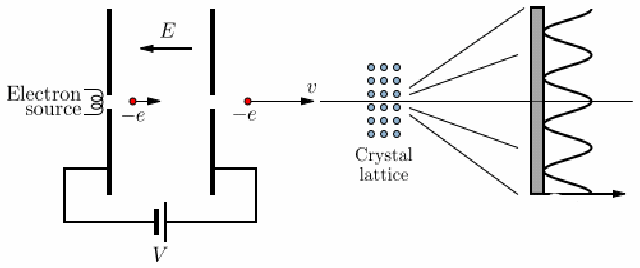
-
using this, get the deBroglie relationship
Wavefunction, wave vector, and Schrödinger equation
-
for an electromagnetic wave:
$$ E_y (x,t) = E_o sin(kx - \omega t) $$
-
for an electron wave:
$$ \psi = A sin(kx - \omega t) $$
$$ \psi = Ae^{j(kx - \omega t)} $$
-
where:
-
k = 2π/λ - the wave vector
-
ω = 2πf
-
A = constant
-
can separate the time-dependent and space-dependent parts and write:
$$ \psi = Ae^{jkx}e^{-j\omega t}$$
-
wave function related to the
probability
of finding the electron at a given point in space and time
-
represents the distribution of the electron wave in time
-
Probability = ΨΨ* = |Ψ|
2
-
probability is a real value
Wave Vector and Potential Energy
-
electron wave momentum is related to the wavelength by this equation: p = h/v
-
momentum is a vector - therefore we need a vector form of the wavelength
-
wave vector:
-
direction: direction of wave travel
-
magnitude: k = 2π\λ
-
momentum now written as:
$$ p = \frac{h}{2π}k$$
-
kinetic energy is:
$$ E_k = \frac{p^2}{2m} = \frac{h^2}{8π^2}\frac{k^2}{m}$$
-
electron also has electrostatic potential energy
-
defined as the work done in pulling the negatively chargely electron from an infinite distance to a distance, r, from the positively charged nucleus:
$$ E_p = \frac{-e^2}{4πε_0 r} $$
-total energy is E = E
k
+ E
p
$$ k = \frac{2π}{h} \sqrt{2m(E - E_p)} $$
Schrodinger’s Equation
-
describes the electron wave function
-
if you know the electron potential energy and boundary conditions, you can calculate the parameters of electron orbital (wave function and energy)
$$ \frac{d^2 \Psi}{dx^2} + \frac{8\pi^2 m}{h^2}(E - E_p )\Psi = 0 $$
“Electron in a box” problem
Electron in a 1D Potential Well
-setup:
- inside the box, potential energy is zero
- outside, is infinitely large
- we need to find the wave equation
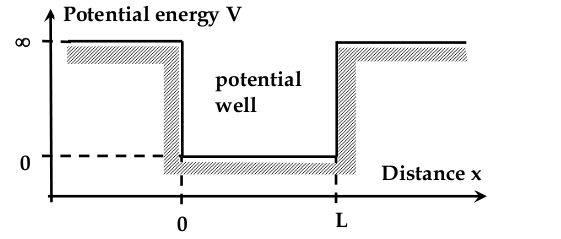
$$ \frac{d^2 \Psi}{dx^2} + \frac{8\pi^2 m}{h^2}(E - V)\Psi = 0 $$
-
assumptions:
-
the case is time-independent
-
wave function is continuous, smooth, and single-
-
Boundary conditions:
-
For x<0 and x>L, the term Vψ dominates
$$ -V\Psi = 0 \\
\Psi = 0
|\Psi|^2 = 0
$$
electron cannot be outside the well
-
Since d
2
ψ/dx
2
must be continuous, ψ = 0 at x={0,L}
-
Differential equation:
-
second order differential equation to solve for within the well
-
general solution equation is:
$$ \Psi (x) = 2Ajsin(\frac{n\pi x}{L}) $$
-
can solve for A because we know the probability of electron being in the box is 1 (integral of equation from 0 to L is 1)
-
final form of the equation is:
$$ \Psi (x) = j(\frac{2}{L})^{\frac{1}{2}}sin(\frac{n\pi x}{L}) $$
Electron Energy in Potential Well
$$ E = \frac{h^2 n^2}{8mL^2} $$
- energies E(n) are the eigenenergies of the electron
- energy is quantized
- n is the
quantum number
- min energy is at n=1, this is the
ground state
- energy of electron wave can only have discrete values
- energy of electron particle can take any value
Uncertainty Principle
-
free electron:
-
has single energy, momentum, wavelength - Δp = 0 (uncertainty 0)
-
electron wave is spread all over the space, so Δx = ∞
-
electron in a potential well:
-
Δx = L
-
Δp = hk/π
-
for n=1, k: = π, Δp = h/L
$$ \Delta x \Delta p = L \frac{h}{L} = h $$
-
Heinsenberg’s uncertainty principle: we cannot simultaneously and exactly know both the position and momentum of an electron along a given co-ordinate
Tunnelling
-
important application of the uncertainty principle
-
if an electron of energy E meets a potential energy barrier of height V_o_ > E, it might leak (“tunnel”) throug the barrier
-
probability of that depends on the energy and width of the barrier
Hydrogen atom
-
consider the H atom: an electron attached to a nucleus
-
electron is electrostatically bound to a single proton
-
since proton is so big, it behaves more like a particle
-
potential energy:
$$ V(r) = \frac{-Ze^2}{4\pi \epsilon_o r} $$
-
where:
-
Z = number of electrons
-
r = (x
2
+ y
2
2 + z
2
)
1/2
-
considerng electron in H atom as confined in a potential well with PE V(r), electron’s wave function can be derived to be:
$$ E = \frac{-Z^2 e^4 m}{8h^2 \epsilon_o^2}\frac{1}{n^2} $$
-
different energy values (different values on) are called
Energy Levels
Atomic Spectra
-
electrons can be excited into higher energy levels - requires energy
-
they can also return to a lower levels - releases energy in the form of a photon with appropriate energy E = hf = E
higher
- E
lower
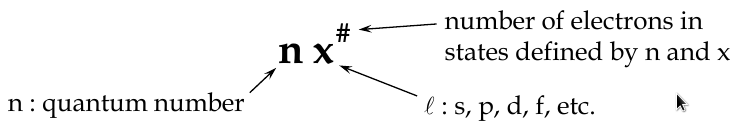
Quantum Numbers
Principal Quantum Number, n
-
determines the radius of electron orbit and the energy level
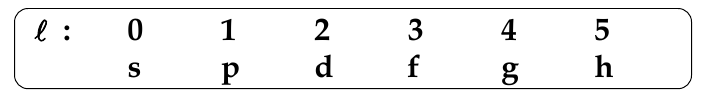
Orbital Angular Quantum Number, l
-
determines the shape of the orbital
-
the electron wave at each orbit (at each r) may be standing or moving along the orbit
-
wave must be continuous and smoothly varying
-
must fit an integral number of wavelengths: lλ = 2πr
$$ L = pr = \ell \frac{h}{2\pi} $$
-
L is the angular momentum, which is quantized.
-
l can take any value from 0 to (n-1)
Magnetic Quantum Number, m
l
-
determines orientation of the orbital in space (the tilt of the electron cloud), and the energy of its electron in a magnetic field
-
angular momentum about the electron orbit is quantized as:
$$ L_z = \frac{m_\ell h}{2\pi} $$
-
-l ≤ m
l
≤ l
Electron Spin Quantum Number, m
s
-
determines the rotation of electron about its own axis
-
has the values 1/2, -1/2 (spin up, spin down)
The Full Sert of Quantum Numbers and Values
n = 1, 2, 3, … z
l = 0, 1, 2, 3, … (z-1)
m
l
= 0, ±1, ±2, ±3, … ±(z-1)
m
s
= ±1/2
Summary of Quantum Numbers
-
Radius of orbit → n
-
Orbital angular momentum → l
-
Tilt of orbit’s plane → m
l
-
Spin of electron → m
s
Electron Clouds
-
we can define electron clouds corresponding to different combos of quantum numbers
-
probability density distribution
Multi-electron atoms, Pauli principle, and the periodic table
Band Structure
Hydrogen molecule and molecular bonding
-
when atoms interact, they change behaviour
-
no two electrons in an interacting systems may occupy same quantum state
-
consider the case of two H atoms
-
when they are infinitely far apart, they have the same wave function
-
when they approach each other, their wave functions overlap and two new
molecular wave functions
emerge (see the image below for the two new functions
-
molecular wave functions are linear combinations of atomic orbitals; in this case, one is the sum and one is the difference
-
Ψ
σ
is more confined to the nuclei, whereas Ψ
σ*
is more spread
-
thus, Ψ
σ*
has higher energy
-
Ψ
σ
then is more energetically favourable, so both electrons occupy this state
-
bonding orbital:
the wave function Ψ
σ
corresponding to the lowest energy level
-
antibonding orbital:
Ψ
σ*
-
total energy of two electrons in H
2
molecule is lower than in two single H atoms
-
one electron has to flip its electron spin but the energy gain due to dropping to bonding orbital is higher than the energy spent
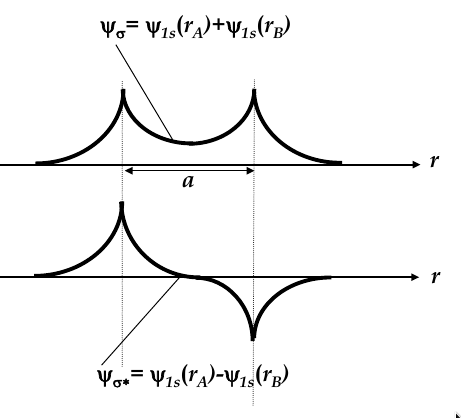
-
consider 3 hydrogen atoms. They will also add their atomic wave functions, like so:
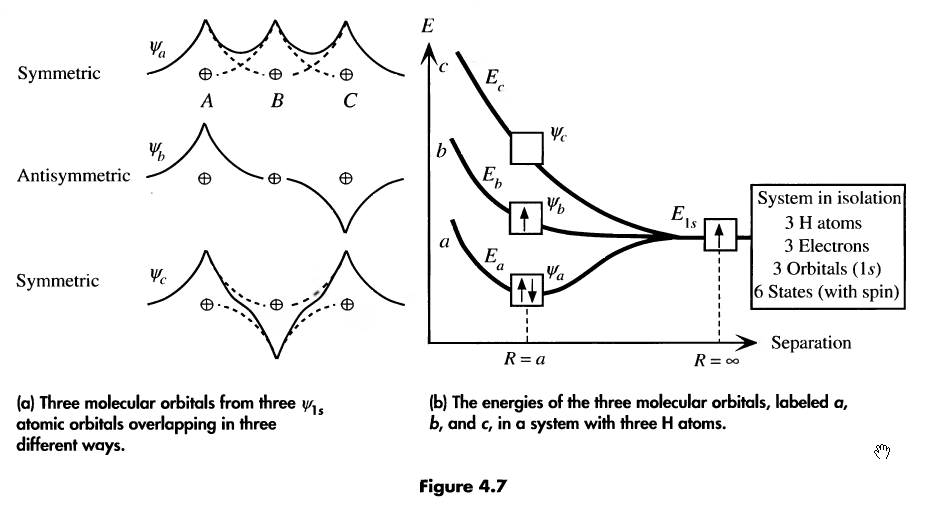
-
the more atoms in our function, the more molecular orbitals they’ll form. n atoms = n orbitals
-
if an energy band is not entirely filled, there are states available for electrons. Consider N Li atoms (2s half filled)
-
thermal energy is enough at room temp for electrons to jump between nearest energy levels
-
since the levels may belong to different atoms, electrons can easily travel from atom to atom
conducting current
Hybridization
-
2s and 2p energy levels are close, so when they approach each other, 2 2s and 2 2p orbitals can mix to form hybrid orbitals
-
hybrid orbitals directed in tetrahedral directions and have the same energy
-
process called
sp
3
hybridization
Energy Bands
-
when interatomic distance decreases so that electrons interact, their energy levels broadens (splits) into bands
-
there are 2N states in the 2s-band, 6N states in 2p-band
-
in diamond crystal, bonding and anti-bonding orbitals split and form
valence band
and
conduction band
, respectively
-
band gap - E
G
- the difference in energy between the conduction and valence bands
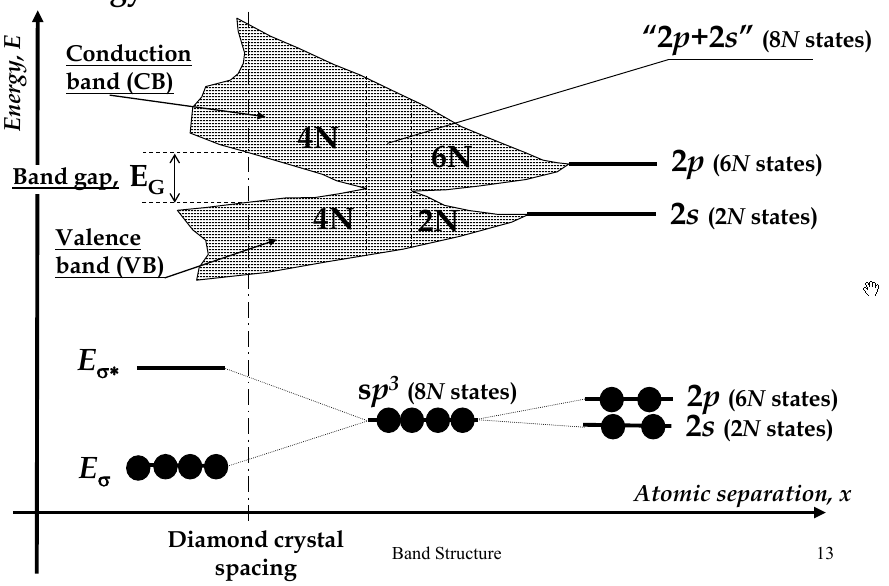
Fermi Energy
-
at T=0K, all electrons will occupy states with lowest energy (valence band), so conduction band empty
-
fermi energy (E
F
) = energy level corresponding to highest filled electron state at 0K.
-
as T increases, bands above E
F
start to get filled
-
to conduct electric current, there must be vacant states in the band
-
no states available in energy levels within each band, no conduction
need more but confusing tho
Effective mass
-
acceleration of an free electron in vacuum is a = F
ext
/ m
e
, m
e
= electron mass in vacuum
-
in a solid, electron interacts with crystal lattice atoms and experiences internal forces F
int
-
thus, acceleration is: a = (F
ext
+ F
int
) / m
e
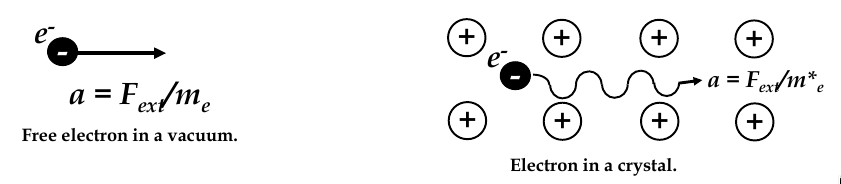
-
since atoms in a crystalline solid are periodically positioned, variation of F
int
is also periodic, we can simplify our acceleration equation:
$$ a_crystal = \frac{F_ext}{m_e^*}
-
where m
e
2
is the
effective mass
of the electron
-
effective mass depends on the material
Electrons and holes
-
in semi-conductors, in order to get excited to empty states, electrons jump across the band gap
-
when excited to the conduction band, a vacant state is left in the valence band
-
this is called a
hole
- the absence of an electron
-
electrical conduction in a semiconductor involves movement of electrons in the conduction band and holes in valence band
-
electron and hole currents
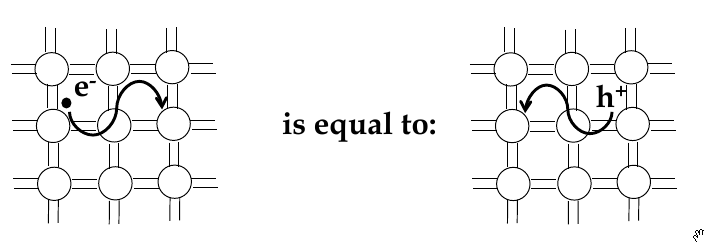
Intrinsic Semiconductor
-
a pure semiconductor (no foreign atoms present) is an intrinsic semiconductor
-
electrons and holes can only be created in pairs (electron-hole pairs)
Carrier Generation
-
electron-hole pair generation: the act of exciting an electron from the valence band to the conduction band
-
electrons can be excited even though E
T
is much smaller than E
G
because atoms in the crystal are constantly vibrating (due to thermal energy) and
deforming interatomic bonds
-
thus, some bonds may be overstretched, and the bond energy can be smaller than thermal energy
-
electron concentration in the conduction band, n, (electrons per cm
3
) is always equal to hole concentration in the valence band, p, (holes per cm
3
)
-
n = p = n
i
-
n
i
= intrinsic carrier concentration
-
g
i
: rate of generation
Recombination
-
opposite of carrier generation: the act of an electron falling back to VB
-
excess energy is released in the form of heat or light
-
rate of recombination, r
i
, is proportional to equilibrium concentration of electrons/holes
-
r
i
= αn
0
p
0
= αn
i
2
-
α = constant
-
n
0
= equilibrium electron concentration
-
p
0
= equilibrium hole concentration
-
in steady state, r
i
= g
i
Conduction
-
takes place only when electron-hole pairs are created
-
conduction not great in intrinsic semiconductors at room temperature
Doping, extrinsic semiconductors
-
doping:
creation of carriers in semiconductors by introducing impurities
-
we get extra carriers, and better conductivity
-
doped semiconductor = extrinsic semiconductor
-
n-type semiconductor:
predominant electron concentration
-
p-type semiconductor:
predominant hole concentration
n-type doping
-
n-type Si obtained by adding small amounts of group V elements (P, As, Sb)
-
these elements have 5 valence electrons, but the atoms bond to Si (4 e
-
), so one of the electrons is
weakly
bonded to the impurity atom
-
very tiny amount of energy needed to excite electrons, so at most temperatures most of the donor electrons will be ionized
p-type doping
-
p-type Si obtained by adding small amount of group III elements (B, Al, Ga, In)
-
these elements have 3 valence electrons, atoms bond to Si (4 e
-
), one of the bonds will miss an electron
-
impurity atoms = acceptors (accept an extra electron)
Carrier concentration
-
how to calculate the number of electrons and holes available for conduction? need to know:
-
number of states available at a particular energy to be occupied
-
fraction of these states that are in fact occupied at a particular temperature
$$ n_o = \int_{E_c}^\infty \! f(E)N(E) \, \mathrm{d}E. $$
-
where:
-
N(E) - density of states
-
f(E) - Fermi function
Density of States
-
DOS: number of available states per unit volume
-
expressions for valence and conduction band are:
$$ N_V(E) = \frac{8\sqrt{2}\pi}{h^3}(m_p^*)^{\frac{3}{2}}(E_V - E)^{\frac{1}{2}} for E < E_V \\
N_V(E) = \frac{8\sqrt{2}\pi}{h^3}(m_n^*)^{\frac{3}{2}}(E - E_C)^{\frac{1}{2}} for E > E_C
$$
Fermi function
$$ f(E) = \frac{1}{1 + e^{\frac{E - E_F}{kT}}} $$
- Fermi-Dirac distribution function gives us the probability that an available energy state at E will be occupied by an electron at temperature T
-
probability that an available energy state will be occupied by a hole is 1 - f(E)
- at E=E
F
, f(E) = 1/2
- E
F
in intrinsic material: middle of band gap b/c concentration of holes in VB = concentration of electrons in CB
- E
F
in n-type material: closer to E
C
because the concentration of electrons in CB is higher than concentration of holes in VB
- E
F
in p-type material: close to E
V
because concentration of holes in VB is higher than concentration of electrons in CB
Equilibrium Carrier Concentration
-
for equilibrium conditions, can use the
effective density of states
* N
C
at energy E_C. Thus:
$$ n_0 = N_C f(E_C) $$
-
Then, f(E
C
) can be expressed as:
$$ f(E_C) = \frac{1}{1 + e^{\frac{E_C - E_F}{kT}}} = e^{-\frac{E_C - E_F}{kT}} $$
$$n_0 = N_C e^{-\frac{E_C - E_F}{kT}} $$
-
similarly, concentration of holes is:
$$ p_0 = N_V [ 1 - f(E_V) ] $$
-
where N
V
is the effective density of states in the valence band
-
$$ p_0 = N_V e^{-\frac{E_F - E_V}{kT}} $$
Mass Action Law
$$ n_0 p_0 = n_i^2 \\
n_0 = n_i e^{\frac{E_F - E_i}{kT}} \\
p_0 = n_i e^{\frac{E_i - E_F}{kT}} $$
Conductivity and mobility
-
current of electrons and holes depends on:
-
carrier concentration (n, p)
-
carrier speed (v
n
, v
p
)
-
carrier charge (q or e)
-
current density can be written as:
$$ J_n = nev_n \\
J_p = pqv_p $$
-
at low electric field, the carrier velocity is proportional to the field: υ = με
-
the proportionality constant μ is called the
mobility
-
total current density is: J = σε
-
ε is called the
conductivity
Hall Effect
-
mobility in semiconductors can be estimated using the Hall effect
-
if we apply electric field E
x
in direction x across a semiconductor and submit it to magnetic field B
z
in direction z, then another electric field E
y
(Hall field) occurs perpendicular to both E
x
and B
z
-
E
y
occurs due to deflection of electrons from direction z due to
Lorentz force
F
y
= -ev
x
B
-
electron velocity in x-direction: v
x
= μ
x
E
x
-
in steady state, deflection is steady and Hall field counterbalances Lorentz force:
-
eE
H
= ev
x
B
z
-
eE
H
= J
x
B
z
/n
-
E
H
/J
x
B
z
= 1/en = R
H
- Hall coefficient
-
μ = | σR
H
| - Hall mobility
Haynes-Shockley Experiment
-
direct way of measuring mobility
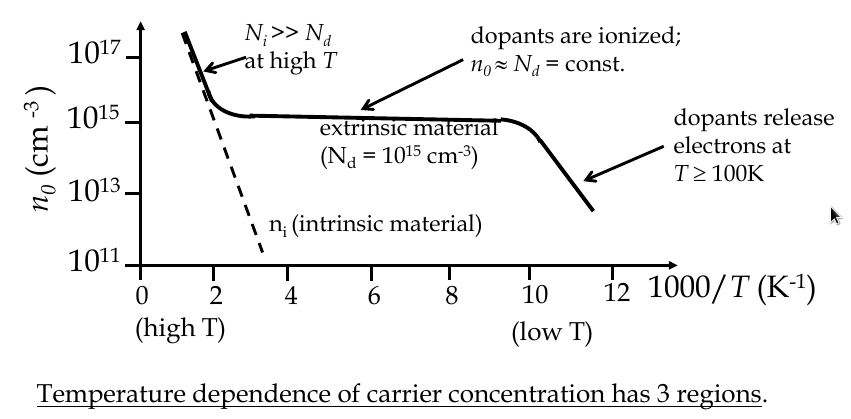
Temperature dependence of carrier concentration
$$ n_i (T) = 2{\frac{2\pi kT}{h^2}}^{\frac{3}{2}}{m_n^* m_p^*}^{\frac{3}{4}}e^{\frac{-E_G}{2kT}} $$
Compensation doping
-
semiconductor could have both acceptors and donors in it: this is compensation doping
-
the concentrations of electrons, holes, donors and acceptors can be obtained from
space charge neutrality law
-
the material must remain electrical neutral overall
-
p
0
+ N
d
+
= n
0
+ N
a
-
-
a material doped equally with donors and acceptors becomes “intrinsic” again
Diffusion Current
-
diffusion: net motion of carriers from regions of high carrier concentration to low carrier concentration if there is non-uniformity (gradient) of carrier concentration
need more
Direction and indirect bandgap semiconductors
-
dielectrics and semi-conductors behave essentially the same way - the only difference is the
bandgap width
-
photons with energy exceeding E
g
are absorbed by giving their energy to electron-hole pairs
-
may or may not reemit the light during recombination depending on whether the gap is
direct
or
indirect
-
direct bandgap
semiconductors: electron drops from bottom of CB to top of VB, excess energy emitted as a photon
-
also known as
radiative recombination
-
indirect bandgap
semiconductors: recombination occurs in two stages via recombination centres (usually defects) in the bandgap:
-
electron falls from bottom of CB to the defect level, then down to the top of VB
-
electron energy is therefore lost in two portions by the emission of
phonons
(lattice vibrations)
-
this process is also known as
non-radiative recombination
Photoconductivity
-
increase of conductivity under illumination
$$ \Delta \sigma = \sigma_photo - \sigma_dark = \frac{e\eta I\lamda \tau (\mu_e + \mu_h)}{hcD}
-
η is quantum efficiency, and τ is average excess carrier lifetime
Energy-band diagrams and MOSFET
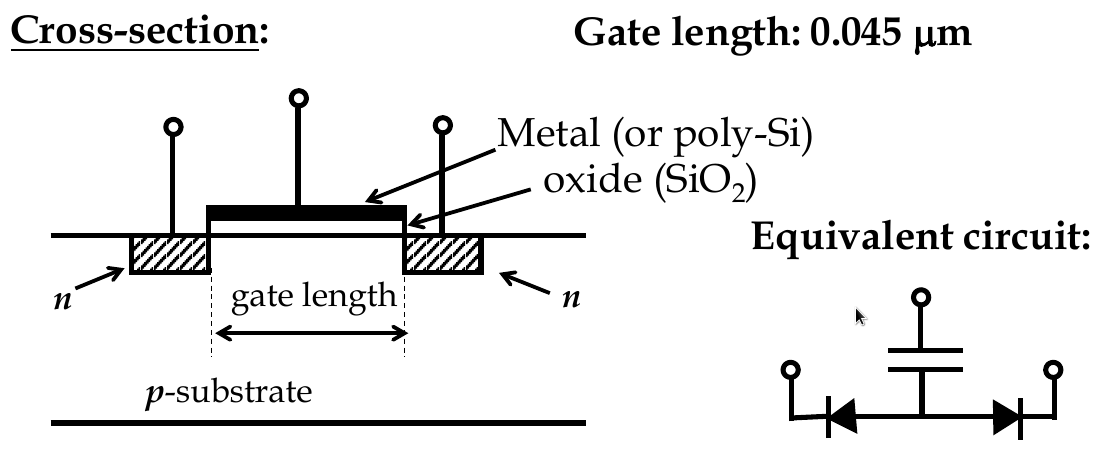
-
no current from source to drain b/c diodes
-
channel is conductive because gate electrode is used
-
there is an insulator between metal and semiconductor, electric field builds across oxide layer if V is applied
-
similar to parallel plate capacitor
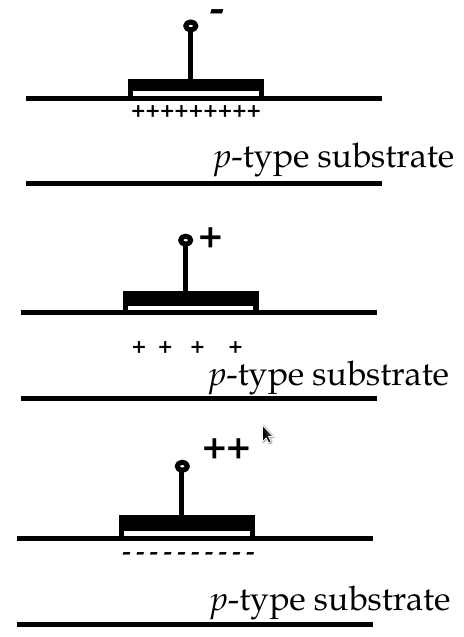
MOSFET Operation Modes
-
accumulation mode
-
negative voltage at the gate increases number of holes at interface
-
depletion mode
-
small positive voltage repels holes
-
inversion mode
-
large positive voltage attracts electrons to the interface, making it locally n-type
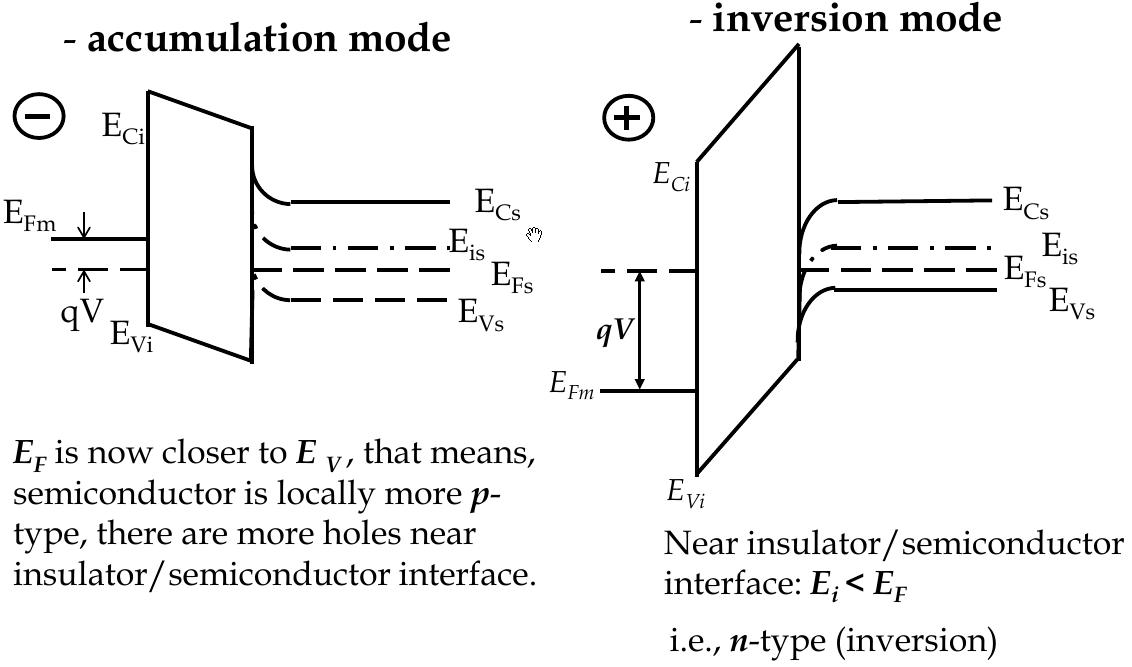
MOSFET Band Diagrams
-
to build energy band diagrams:
-
choose zero points on co-ordinates (for energy axis, 0 is vacuum level)
-
in equilibrium, E
F
= const everywhere on band diagram xo
-
more devices per unit area - more metal interconnection lines
-
issues:
-
longer interconnects
-
higher capacitance per unit area
-
increasing heat (more devices per chip and higher frequency => increased heat production)
-
interconnects: resistance is R =ρl/A, resistance increases as width/length decrease
-
capacitance: metal lines end at MOSFET gate, forming RC line - potential source of slowing down the circuit speed
-
heat production: at junctions between metal layers, metal is thin => resistance higher, higher heat production
-
metal atoms have 1 to 3 valence electrons and ionize easily
-
electrons are shared between all atoms, so metal ions are surrounded by electrons
-
electrostatic forces are equal in all directions
-
ions positions are fixed
-
electrons move around freely
-
in a perfect metal crystal at T=0, there is
no resistance
due to the wave nature of electrons
-
an electron moving at constant velocity behaves as a plane wave
-
after interactions with wave, ions become the “sources” of secondary wavelets
$$ n\lambda = 2dsin\theta $$
-
in case of small interatomic distance and low electron speed
$$ \lambda > 2d \\
\frac{\lambda}{2d} = \frac{sin\theta}{n} > 1 $$
-
only solution is at n=0, θ=0
-
transmission occurs in direction of travel, magnitude unchanged
-
source of resistivity is either the
temperature
or
non-crystallinity
of a metal
Temperature dependence of resistivity
-
At T>0K, atoms move away from ideal lattice position b/c vibrations
-
electrons become scattered
-
for atoms in a gas:
-
mean free path:
average electron path length is defined
-
mean free time:
time between collisions
In the Presence of an Electric Field
-
when a potential difference is applied across metal, electrons drift towards larger positive potential
-
current density:
$$ J = nqv^d$$
Drift Velocity
-
in electric field, electrons experience acceleration
-
electron collisions with lattice ions causes velocity loss
-
net acceleration of electrons between collisions
$$ \frac{dv_d}{dt)_ACC = frac{-q\epsilon}{m} $$
-
velocity loss at each collision:
$$ \frac{dv_d}{dt}_LOSS = \frac{-v_d}{\tau} $$
$$ \frac{dv_d}{dt}_TOTAL = \frac{v_d}{\tau} + frac{-q\epsilon}{m} = 0 $$
-
mobility: μ = qτ/m
Phonons
-
due to bonding, atom motions are connected (behave like a wave)
-
types of wave motion:
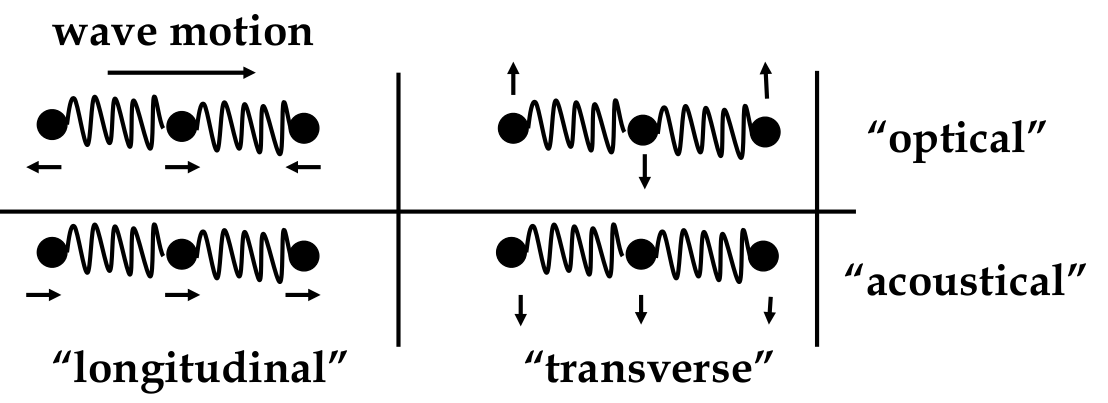
-
these waves act like
phonons
-
can model interaction between lattice and electron wave as interaction between electron and phonon
Structural dependence of resistivity
-
structural disorder gives rise to resistivity
-
list of imperfections in a chip includes:
-
impurity atoms
-
dislocations
-
grain boundaries
Superconductivity
-
for many elemental metals and alloys, the resistivity falls to an immeasurably small value at some point before the critical temperature (T
C
)
The Meissner Effect
-
when superconducting material at temperature above T
C
is placed in a magnetic field and then cooled down, all magnetic field lines are ejected from the material at T=T
C
Optical Properties
Light wave propagation, Refraction Index
-
the velocity of the wavefront of light depends on the material in which it is travelling (because waves, yo)
-
in dielectric non-magnetic material, electric field part of the wave interacts with electrons etc, polarizes atoms and molecules at the frequency of the wave
-
since wave propagation is coupled with dipole formation, polarization slows down propagation
-
rate of propagation is characterized by dielectric permittivity and magnetic permeability
$$ v = \frac{1}{\epsilon_r \epsilon_0 \nu_r \nu_0} $$
Refraction Index
-
refraction:
the bending of light as it passes from one material to another (due to the change in velocity)
-
refraction index is
n = c/v
, v = speed of light in material
-
is a consequence of electric polarization
-
when a light wave passes through a material, energy is lost to the electrons of the material
-
energy transferred comes from the velocity change
-
since polarization is frequency dependent, refractive index also depends on the wavelength of light
Dispersion
-
dispersion:
a general name give to effects that vary with wavelength
-
wavelength dependence of the refractive index is the
dispersion of the refraction index
$$ n^2 = 1 + \frac{A_1 \lambda^2}{\lambda^2 - \lambda_1^2} + \frac{A_2 \lambda^2}{\lambda^2 - \lambda_2^2} + ... } $$
-
A
n
and λ
n
are the
Sellmeier coefficients
-
since white light is a collection of multiple wavelengths, its velocity is a
group velocity
, and has a
group index
Snell’s Law, Total Internal Refraction
-
angles of incidence and refraction are related by Snell’s Law
$$ \frac{sin\theta_1}{sin\theta_2} = \frac{v_1}{v_2} = \frac{n_2}{n_1} $$
-
in the case n
2
< n
1
, refraction angle θ
2
exceeds 90º, the light does not exit material 1 but is
totally internally reflected
-
respective incidence angle is called critical angle θ
c
$$ sin\theta_c = \frac{n_2}{n_1} $$
Optical Fibers
-
got that total internal reflection going on
-
fiber optic tech has super fast speed of data transmission
-
HELP
Photon interaction with materials. Absorption, Reflection, Transmission, Refraction
-from conservation of light, I
0
= I
T
+ I
A
+ I
R
,
- I
0
is the intensity of incident light, I
T
, I
A
, I
R
are intensity of transmitted, absorbed, and reflected light
- three types of light-material interactions:
- transmission
- absorption
- reflection
- materials divided into:
- transparent (little absorption and reflection)
- translucent (light scattered within material)
- opaque (relatively little transmission)
- if material not perfectly transparent, light intensity decreases exponentially with distance
- if the light intensity drop in δx is δI δI = -α δx I
- α = absorption co-efficient (m
-1
)
Bouger-Lambert-Beer’s Law
$$ ax = -ln(frac{I}{I_0}) $$
- light could be absorbed by the nuclei (all materials) or by the electrons (metals and narrow E
g
semiconductors)
Atomic Absorption
-
type of absorption strongly depends on the type of material that absorbs
-
ionically bonded solids show high absorption because oppositely charged ions move in opposite directions creating more interactions
-
phonons exist in bands but only one of the phonon energies is excited by the radiation, so there is only one absorption frequency
-
transmission spectrum shows just one dark and is called
line spectrum
-
absorption spectrum is dominated by the absorption due to the molecules themselves
-
air pollution monitoring: can fitting known spectra of various gases to the measured atmospheric spectra over the same frequency range
-
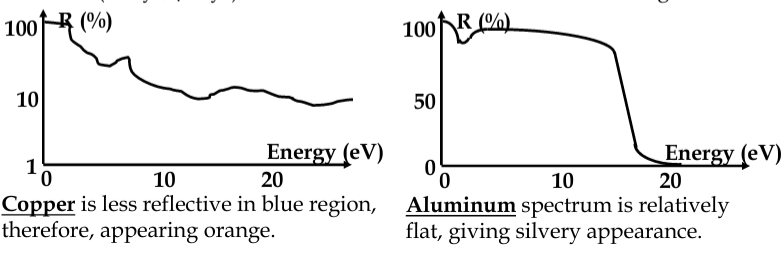
in metals, photons are absorbed by electrons
-
almost any frequency of light is absorbed
-
practically all light absorbed within about 100nm of metal surface; thinner metal films will partially transmit light
-
excited electrons in the surface layers of metal - recombine again, emitting the light
-
metals are both opaque and reflective
-
reflection can be explained in terms of electrostatics
-
EM field forces the free electrons to move, moving charge is source of EM waves. Therefore, wave is reflected
-
band structure of metals not as simple as we assumed - there can be absorption below E
F
-
metals are more transparent to very high energy radiation, where the inertia of electrons is the limiting factor
-
dielectrics and semiconductors behave essentially the same way - only difference is the
bandgap width
-
photons with energy exceeding E
g
are absorbed by giving their energy to electron-hole pairs
-
may or may not re-emit the light during the recombination, depending on whether the gap is direct or indirect
-
direct bandgap:
excess energy emitted as a photon
-
indirect bandgap:
energy is lost in two portions by the emission of
phonon
(lattice vibration)
Reflection
-
occurs at the interface between two materials and is therefore related to refraction index
-
reflectivity is the ratio of incident and reflected light intensities
-
assuming light is incident normally to the interface
$$ R = \frac{n_2 - n_1}{n_2 + n_1}^2 $$
Transmission
-
reflection and absorption are wavelength dependent
-
transmission - a “leftover” after reflection and absorption
-
to get transmission spectrum, just subtract reflection and absorption spectra from the incident light spectrum
$$ I_T = I_0 - I_A - I_R $$
-
small differences in composition may lead to large differences in appearance
Colours
-
Al
2
O
3
(sapphire) is colourless - adding 0.5-2.0% of Cr
2
O
3
turns the material red - ruby!
-
Cr atoms substitute Al in the crystalline lattice and introduce impurity levels in sapphire bandgap
-
these levels give strong absorption at 400nm (violet) and 600nm (orange), leaving only red light to go through
-
similar technique is used to colour glasses by adding impurities while in the molten state
Emission. Luminescene and Fluroscence
-
luminescence:
general term which describes the re-emission of previously absorbed radiative energy
-
common types: photo-, electro-, cathodoluminescence
-
depends on source of incident radiation: light,(fluorescent light) electric field (LED), or electrons (CRT)
-
also chemoluminescence due to chemical reactions (which makes glow sticks)
-
luminescence is further divided into
phosphorescence
and
fluorescence
-
fluroescence: electron transitions that require no change of spin
-
phosphorescence: electron transitions that require a change of spin
-
hence, fluroescence is faster!
Luminescence
-
if the energy levels are actually a range of energies, after electron excitation we observe a series of transitions accompanied by phonon emission, and then fluorescent transition
-
since part of electron energy is released as phonons, then the light emitted by fluorescence is of longer wavelength than incident light
Lasers
-
LASER: Light Amplification by the Stimulated Emission of Radiation
-
before, we considered spontaneous light emission, which happened due to randomly occurring effects
-
stimulated emission refers to electron transmissions that are stimulated by the presence of other potons
-
an incident photon with E >= E
g
is equally likely to cause stimulated emission of another photon as be absorbed
-
emitted photon has the same energy and phase as the incident photon (i.e. they are coherent)
-
normally we have less electrons in the excited state than ground state
-
if we somehow get more electrons in the excited state than ground state, than we get stimulated emission -> much more photons in the output than in the input -> we get
amplification
-
population inversion:
when we have more electrons in the excited state than in the ground state
-
since random spontaneous emission gives incoherent output, it should be minimized in LASERs
-
done by using transmissions from which spontaneous emission is less likely; transmission from
metastable
states

-
common material for solid state lasers is ruby, sapphire with Cr impurities
-
to make laser, we have to achieve
-
population inversion
-
enough photons to stimulate emission
-
first condition is met by filling the metastable states with electrons using a zenon flash lamp (in ruby laser) or by electron injection (in semiconductor laser)
-
second condition is achieved by making laser in the rod shape. By mirroring the ends of the rod, we let photons travel back and forth along the rod
-
in order to keep the coherent emission, we must ensure that the light completes the round trip between the mirrors and returns in phase with itself
-
in order to produce coherent output, the distance between the rod ends must obey the relationship:
nλ= 2L
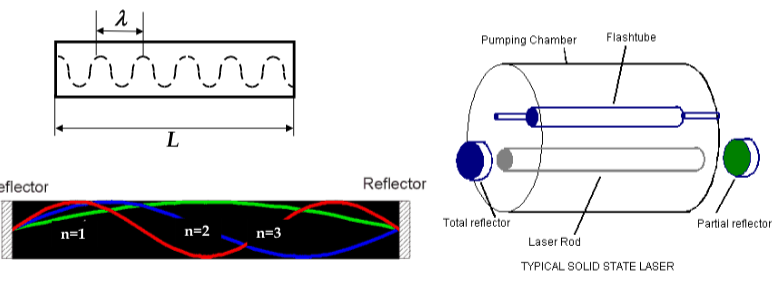
-
in semiconductor lasers, thin films of direct semiconductors are epitaxially deposited on top of each other
-
central layer is degenerately doped (the doping is so heavy that E
F
< E
V
and there are lots of empty electron states in the valence band)
-
under bias, electrons are injected from n-layer into central later and get trapped there
-
thus, population inversion in central layer
Dielectric Materials
-
dielectrics increase the capacitance between parallel plates
$$ C + \frac{\epsilon_o \epsilon_r A}{d}
-
increased capacitance allows more charge storage
-
why do we care about studying dielectric materials?
-
electron devices become smaller! A is always decreasing as the devices shrink; d cannot decrease indefinitely as bellow 5-10nm, tunnelling occurs. Therefore you get less charged stored as devices shrink.
-
but you cannot increase V too much as breakdown will occur. So the only way to increase C is to the increase the relative permittivity, which depends on the dielectric
-
types of dielectric materials:
-
MOSFETs: charge has to be accumulated at the semiconductor/gate dielectric interface
-
DRAM: charge is injected and retained in a capacitor with a MOSFET switch
-
CCD cameras: charged generated by photons is retained in the capactiros and read out by charge transfer between them
-
LCD: each pixel is a capacitor; charge stored generates E-field across liquid crystal layer and causes LC molecules to rotate
Surface Charge and Surface Electricity
-
dielectrics are insulators; do no conduct electric current at room temp
-
electric charge deposited on the dielectric surface cannot move and stays on the surface (e.g. being attached to surface defects)
-
dielectrics are therefore efficient in storing electrostatic charge!
Surface Charging
-
electron interactions at surfaces are complex and still poorly understood
-
all materials have “surface states” caused by the incomplete bonds
-
strongly affect the behaviour of many electronic devices
-
all ICs are covered with protective layer (‘passivation’) to prevent contamination by water vapour, etc.
-
many materials that bond covalently or ionically charge positively when rubbed
-
friction removes unbonded electrons
-
however, polymer-based materials tend to charge negatively:
-
consider a material like paraffin. The side-arms of molecular structure tend to attract water. Friction removes the H+ ions, leaving the OH- behind
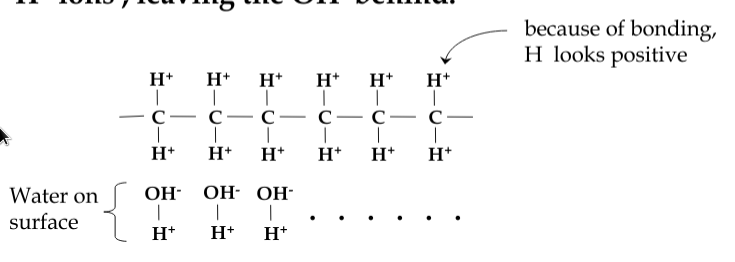
Coulomb’s Law
-
summary of electrostatics:
-
like charges repel, opposites attract
-
the force between charges:
-
inversely proportional to distance
2
-
dependent on surrounding medium
-
acts along a line joining charges
-
proportional to each charge
-
Coulomb’s Law:
$$ F = \frac{q_1 q_2}{4\pi \epsilon r^2} u $$
-
in dielectrics, ideally no electrons available in the conduction band
-
in reality, some electrons are present caused by random phenomenon (e.g. UV rays, cosmic rays)
-
will be a small leakage current
Capacitance Effects: Fringing Fields
-
simple expression for capacitance has ignored several factors - most important being
Edge effects
-
electric field at the edges of a capacitor is non-uniform
-
edge effects are important also when considering the RC time constants of the IC interconnects (higher RC values limit the speed of the ICs)
-
fringing fields increase the effective area of the capacitor, leading to significant error
-
in ICs, also have to consider
capacitive coupling
between metals in different layers causing
crosstalk
Polarization and Relative Permittivity
What Happens: Dielectric Between Plates of a Capacitor
-
more general definition of electric field: E
x
= -dV/dx = -∇V
-
when a dielectric is inserted into parallel plate capacitor, additional charge is being stored on the plates
-
relative permittivity increases as a result
-
increase in the stored charge is due to polarization of the dielectric in the electric field
-
atoms and molecules become
polarized
when they are subjected to an electric field, and form electric dipoles
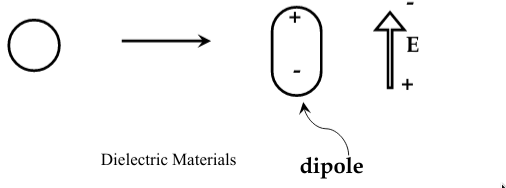
-
polarization occurs when voltage is applied to capacitor - first electron’s worth of charged is induced on the plates
-
the charge causes the dielectric to polarize, and therefore does not contribute to building up the potential difference across the capacitor
-
this same thing happens to the next electron’s worth of charge -> until all atoms in the dielectric are polarized
-
only then due charge induced on capacitor start to contribute to build up the potential difference across the capacitor
-
therefore more charge has to flow in before capacitor charged up to the supply voltage
-
charge storage capacity has increased
-
dielectric has increased its capacitance
Polarization
-
in the presence of electric field, centres of each charge become slightly misplace and the particles become polarized - electric dipoles
-
polarization is equal to the bound charge per unit area of the dielectric surface; measured in Coulombs/m
2
-
dipole moment:
the (absolute) charge on each of the two dipoles separated by their distance
-
consider P bound charges per unit area on opposite sides of a cube with side l, A = l
2
-
oppositely direct dipoles inside the cube cancel out each other; the only uncompensated charge is next to the surfaces of the cube
-
total dipole moment is therefore: μ= P
A
l
-
P is
electric dipole moment per unit volume
-
INSERT MORE HERE 23-24
Polarization Mechanisms
-
dipole moment also depends on the electric field
within
the material
-
remember that zero field gives no polarization, so μ must be field dependent
-
μ= αE
int
-
α =
polarizability
of the material
-
the average dipole moment per unit of internal field
-
Clausius Equation:
-
P = (ε
r
- 1)ε
o
E = N αE
int
-
several mechanisms that contribute to α: α = α
e
+ α
a
+ α
d
+ α
i
-
α
e
: electrical polarizability
-
α
a
, α
d
: molecular polarizability
-
α
i
: interfacial polarizability
Electrical Polarizability
-
also called optical polarizability because the polarization can keep up with even optical frequencies
-
with no electric field, electron clouds are symmetric around the nucleus
-
when field is applied, electron cloud is distorted
-
the centers of the negative and positive charges are now offset, leading to an electric dipole μ
e
= α
e
E
int
Molecular Polarizability
-
arises when the molecules of the material naturally forms dipoles (e.g. H
2
O)
-
two things can happen when an electric field is applied:
-
atomic polarizability α
a
-
orientational polarizability α
d
-
no α
d
in ionically bonded solids because strong bonding forces prevent wholesale re-alignment of molecules
-
however, small change in “centre of mass” of the bonds so a small α
a
can be present

Interfacial Polarizability
-
accounts for the presence of lattice imperfections, ionized contaminants, a few electrons etc.
-
in an electric field, some or all of these can move through the material until they come to an interface
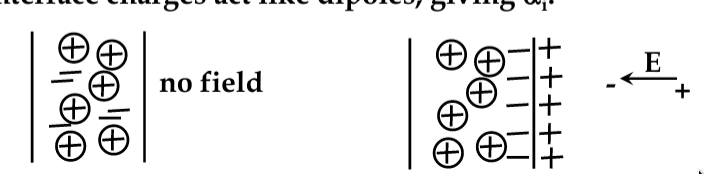
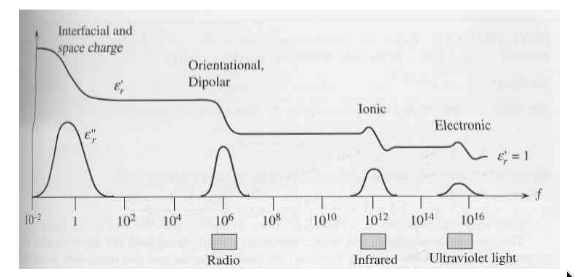
Frequency Dependence of Polarization
-
how do we distinguish the effect from different types of polarization?
-
if you put a dipole in an alternating field, the dipole will attempt to follow the oscillating field
-
but the dipole has inertia, so it takes a finite time to respond to field
-
if we oscillate the field fast enough, the dipole will eventually cease to respond fast enough
-
relaxation frequency:
the frequency at which the dipoles cannot move at all before the field reverses direction (hence the polarization “goes away”)
-
relaxation occurs at different frequencies for different mechanisms of polarization
-
relaxation frequency higher when switching smaller masses of material (less inertia)
-
so if we plot the permittivity as a function of frequency, should find changes at each relaxation frequency
-
electronic: only electrons must be moved, they’re very light so relaxation occurs at high frequencies
-
atomic: relies on ions moving their positions so freq should be about the same as thermal oscillations of the atoms
-
orientational: requires reorganization of groups of dipoles - freq lower than atomic
-
interfacial: caused by charge that percolates slowly through entire thickness of material; very low freq
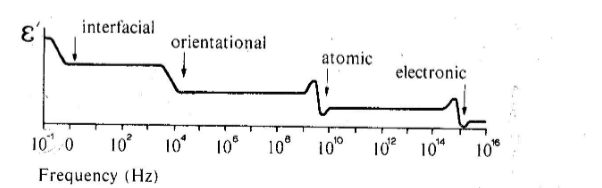
Classification of Dielectrics
-
classify dielectrics into three categories:
-
non-polar materials:
show variations of permittivity in the optical range of frequencies only
-
polar materials:
display both atomic and electric polarizability
-
dipolar materials:
display atomic, electric, and orientational polarizability
AC Permittivity and Dielectric Loss
-
dielectrics may have different response time in case of AC voltage
-
current leads the voltage when voltage applied to capacitor
-
in a real capacitor, there will be a small leakage current through the dielectric, which will be
in phase with V
-
thus, real capacitor consists of a capacitive component connected in parallel with a resistive component
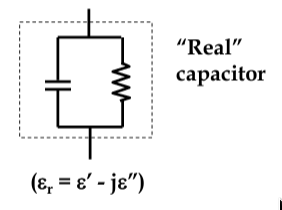
-
since the impedance now consists of real and imaginary components of 0
o
and 90
o
phase, total phase difference is slightly less than 90
o
, by δ
o
-
δ is the
loss angle
-
mathematically, this is accounted for by defining the permittivity to be a complex number:
-
ε
r
= ε
’
- jε
’‘
-
loss angle is therefore tanδ = ε
’‘
/ε
’
-
since power dissipated in the capacitor is proportional to the value of ε
’‘
, dielectrics want as small a δ as possible
-
capacitive component of the capacitance has the same frequency dependence as the polarizability
-
resistive component of the capacitance has several maxima at the frequencies at which capacitive components of the capacitance cease to respond
-
these frequencies (relaxation peaks) should be avoided due to power dissipation
Gauss’ Law and Boundary Conditions
-
what if we have non-uniform dielectric between the plates of the capacitor?
-
electric flux density, D, in the material is given by:
D = εE
-
from Coulomb’s law, we get:
$$ D = \frac{q}{4\pi r^2} a $$
-
this means D is independent of ε for fixed q
-
the total flux crossing the sphere area is given by D*Area
-
this leads to:
$$ Flux = 4\pi r^2 \frac{q}{4\pi r^2} = q $$
-
total flux out of the surface is equal to the enclosed charge; the generalization of this to all closed surfaces is
Gauss’ Law
Dielectric Breakdown
-
we cannot apply an infinitely large voltage across our capacitor without it breaking down
-
dielectric breakdown usually evidenced by a sudden increase in current to a very large value
-
breakdown voltage (V
BD
):
voltage that causes dielectric breakdown
-
dielectric strength (E
BR
):
maximum electric field that can be applied to a dielectric without a breakdown
-
in real insulators, breakdown voltage can be hard to predict since it depends on surface and ambient conditions
Breakdown Mechanisms
-
avalanche breakdown:
occurs if the electric field across the insulator is high enough
-
the few electrons present can achieve enough energy to ionize other atoms (impact ionization)
-
secondary electrons are also accelerated, causing further ionization and avalanche develops
-
thermal breakdown:
occurs if the leakage current (loss angle) is large enough to cause significant heating -> more leakage -> more heating
-
discharge breakdown:
occurs is small gas bubbles are present in the material
Capacitors and Memories
-
main applications of dielectrics in electronics: capacitors and memory cells
-
capacitors:
can be made as discrete elements or be integrated with other elements on the same wafer
-
discrete caps made from alternating layers of metal and dielectric, wrapped up in a package
-
dielectric material is chosen to get the right range of values with the minimum loss angle
RAM
-
same problem of fitting capacitor into a small space occurs with RAM cells
-
in RAM, SiO
2
is used as the dielectric
-
data is stored as the charge in a capacitor cell with a MOSFET as a switch
-
as the devices shrink, the challenge is how to maximize the charge storage and minimize the cell area
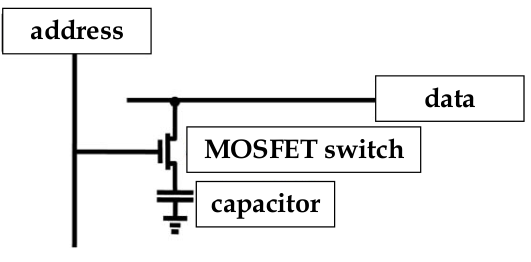
-
reducing cell area is important for increased storage densities
-
chip manufacturers make the capacitors 3D, which requires complex fabbing and may be more susceptible to faults
-
could increase charge storage capacity by using another material but manufacturers are slow to change their fabbing process
Piezoelectric Materials
-
piezeoelectric materials:
a mechanical stress (tension or compression force/area) causes a dielectric polarization or an applied electric field will cause a mechanical strain
-
used for electromechanical sensors and actuators
-
mechanism of piezoelectricity involves an asymmetry in the arrangement of positive and negative ions in the material
-
have a hexagonal unit cell
-
with no pressure applied, the centers of positive and negative charges concide
-
when mechanical pressure is applied in the vertical direction, the centers split - a dipole forms
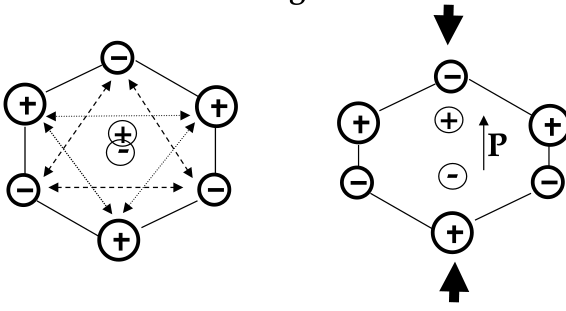
-
symmetric material would not change its dipole moment when stressed
-
pressure applied in the horizontal direction does not induce polarization - piezoresistivity is
anizotropic
-
polarization, P, is related to mechanical stress, T
-
electric stress, E, is related to the mechanical strain, S
$$ d = (\frac { \partial P}{\partial T})_E = (\frac { \partial S}{\partial E})_T = "polarization coefficient" $$
-
quartz is historically the first piezoelectric material
-
quartz crystal is cut into a disc and electrodes are plated onto opposite sides
-
now, the disc has a mechanical resonant frequency precisely determined by its size
-
we can excite mechanical oscillations by applying an AC voltage
-
the resonant frequency of that voltage is therefore the same as that of the mechanical oscillation
Thermal Properties of Materials
Heat Issues in ICs
-
electrons release their energy to vibrating atoms upon collisions, causing heating
-
if generate heat is not removed, temperature increases
-
consequences:
-
carrier mobility may change
-
carrier concentration may change
-
dielectrics may leak more or even break down
-
heat generation in IC due to electric power:
-
P = IV = JAV = V
2
/R = I
2
R
-
total generated heat increases with:
-
more devices or interconnection lines per unit area (higher J)
-
higher operating voltage
-
higher operating frequency
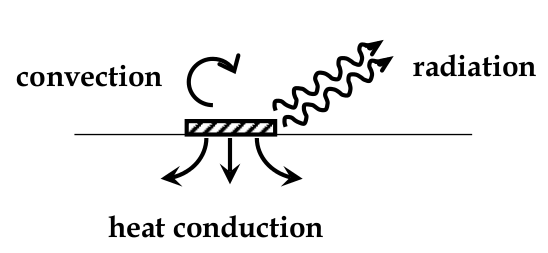
Mechansms of Heat Generation and Loss
-
heat loss mechanisms in IC:
-
heat conduction
-
heat radiation
-
convection
-
heat conduction:
flow of heat through a solid, analogous to electronic transport but with:
-
driving force being ΔT instead of ΔV
-
constant of proportionality is thermal conductivity instead of electrical conductivity
-
heat radiation
: loss of energy by the emission of EM radiation in the IR wavelengths
-
heat convection:
transfer of heat away from a hot object because the gas next to it heats up and becomes less dense and then rises
Overview of Heat
-
heat (Q) flows from hotter objects to cooler
-
First Law of Thermodynamics: ΔE = W + Q
Heat capacity
-
heat capacity (C): ability to absorb heat from the external surroundings; amount of energy needed to heat particular material
-
depends on conditions measured under (volume and pressure)
-
C
P
’
: under constant pressure
-
C
V
’
: under constant volume
$$ C_V^\prime = C_P^\prime - \frac{\alpha TV}{K} $$
-
specific heat capacity:
heat capacity by unit mass
$$ c_p = \frac{C_P^\prime}{m} $$
$$ c_v = \frac{C_V^\prime}{m} $$
-
molar heat capacity:
heat capacity per moles of atom
$$ C_V = c_vM = \frac{C_V^\prime} {n} $$
Dulong-Petit Law
-
C
p
= C
v
= 25 J*mol
-1
K
-1
-
Dulong-Petit Law: heat capacity of metals saturates at 25 J*mol
-1
K
-1
-
heat capacity and thermal conductivity can be calculated by:
-
classical theory based on thermodynamics
-
quantum mechanics based on interaction
Thermal Conductivity
-
thermal conductivity (K):
proportionality coefficient between heat flow density and temperature gradient
-
Fourier’s Law:
$$ J_Q = -K\frac{dT}{dx} $$
-
slightly temperature dependent, usually decreases as temperature increases
Classical treatment of heat capacity
-
treat electrons in a metal as a gas
-
ideal gas law: PV = nRT
-
will use in calculation of kinetic energy of gas molecules (or electrons in a metal)
-
in a small volume of gas, about 1/3 on average will move in x-direction - 1/6 will move in +x direction
-
number of particles per unit time that hit the end of the volume per unit area will be:
$$ Z = \frac{1}{6}n_v v $$
-
where v= velocity, n
v
= particles per unit volume
-
momentum transferred per unit area is:
$$ p^* = Z2mv = \frac{1}{6}n_v mvm = \frac{1}{3}\frac{N}{V}mv^2 $$
-
we find that the average kinetic energy of a gas molecule:
$$ E_kin = \frac{3}{2} kT $$
-
atoms vibrate about their ideal lattice positions due to their thermal energy
-
such an atom can be thought of as being like a sphere supported by springs
-
the atom acts like a simple harmonic oscillator which “stores” an amount of thermal energy
-
in a three-dimensional solid, oscillator has energy E = 3kT; energy per atom
-
total internal energy per mole is therefore:
E = 3N
o
kT
$$ C_V = \frac{\partial E}{\partial T} = 3kN_o $$
- classical treatment works well at high temperatures
- implies that heat capacity is constant and independent of temperature
- however, heat capacity is dependent on temperature
-
Debye Temperature (θ
D
)
: temperature at which C
V
has reached 96% of its final value
Quantum Theory of Heat Capacity
-
key assumption: the energies of the “atomic oscillators” are quantized
-
such quantized lattice oscillations are called
phonons
-
number of phonons increase with temperature
-
energy of each phonon is constant (electrons - number is constant but energy changes)
-
Bose-Einstein Distribution
: the average number of phonons at any temperature was found to obey a distribution
-
turns out, electrons play a small part in the heat capacity; only a small fraction of the total number of electrons can gain thermal energy
-
1% of C
V
is contributed by electrons at room temperature
Quantum treatment of thermal conductivity
-
what is the mechanism of heat transfer?
-
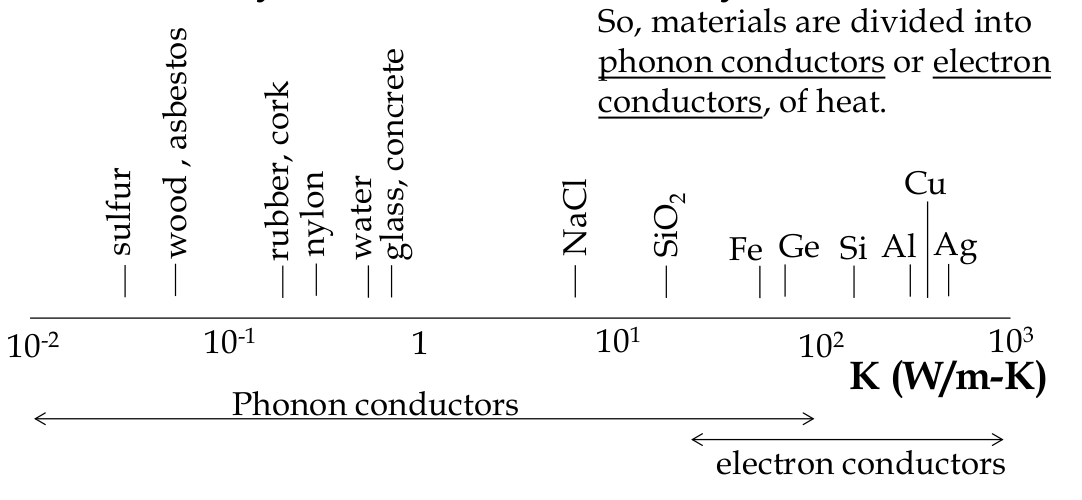
in a solid, only two things can move:
-
depending on the material, either one or the other tends to dominate
-
good electrical conductors tends also to be good thermal conductor
-
Wiedemann-Franz Law:
relationship between electrical and thermal conductivities (for metals), suggesting that
electrons can carry thermal energy as well as electrical
-
because of electrical neutrality, equal number of electrons move from hot -> cold and from cold -> hot
-
but their thermal energies are different and so the heat transported is proportional to the difference between electrical and thermal energies
-
in electrical insulators, there are few free electrons so the heat must be conducted in some other way - phonons (lattice vibrations)
-
the major difference between conduction by electrons and by phonons:
-
electrons: number constant but energy varied
-
phonons: number is variable (more phonons at hot end) but energy is quantized
Classical theory of Thermal Conductivity
-
let us consider a bar of material with a thermal gradient dT\dx
-
we calculate the flow of energy through a volume due to the temperature gradient and use this to calculate the number of “hot” electrons
-
an equal number of “cold” electrons must flow the opposite way, so we can solve for K
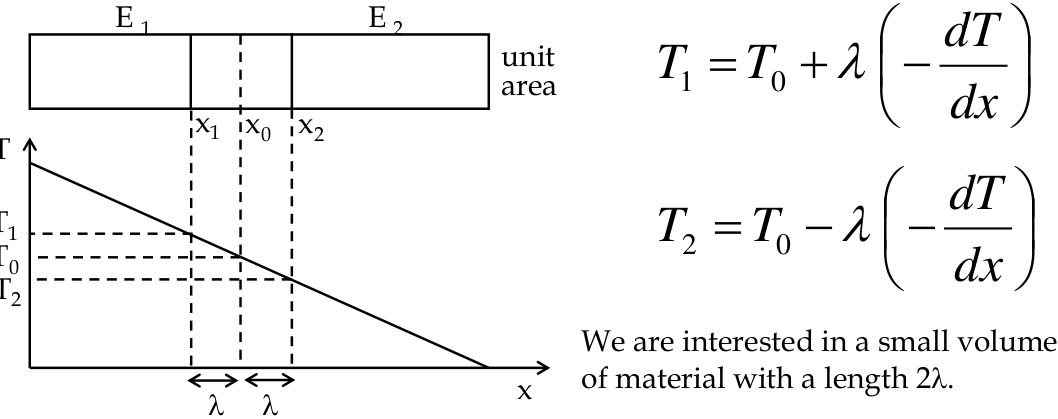
- λ is the
mean free path
between collisions with the lattice
- the idea is that an electron must have undergone a collision in this space and hence will have the energy/temperature of this location
Wiedemann-Franz Law
$$ \frac{K}{\sigma T} = \frac{\pi
2 k
2}{3q^2} = L $$
- L = Lorentz number
- works quite well for metals, but not for “phonon materials”
Thermal resistance
-
just as we can relate electrical conductivity to electrical resistance, we can obtain a thermal resistance
-
for the temperature of the device on the chip:
-
T
j
= T
a
+ θ
ja
P
-
T
j
junction temperature
-
T
a
ambient temperature
-
P dissipated power
-
θ
ja
junction-to-air thermal resistance
-
θ
ja
can be composed of several resistances in series
-
θ
ja
= θ
D
+ θ
j1
+ θ
P
+ θ
j2
-
θ
D
chip
-
θ
j1
chip-to-package
-
θ
P
package
-
θ
j2
package to air
-
would like to minimize θ
ja
in order to get a smaller drop in temperature per unit power
-
recall we can lose heat due to radiation, conduction, and convection
-
all of these depend on surface area, so we can improve heat dissipation by using a high sink of high thermal conductivity
Magnetic Properties
Basic Concepts
Faraday’s Experiment
Para- and Dia-magnetics
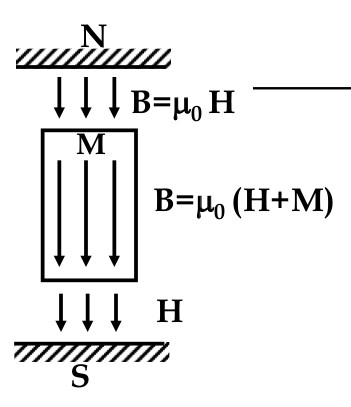
Paramagnetic and Ferromagnetics:
$$ B = \nu H = \nu_0 (H + M) $$
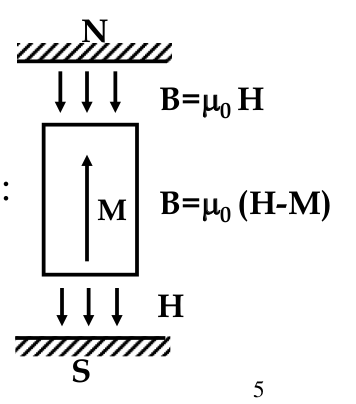
Diamagnetics:
- M opposes H
$$ B = \nu H = \nu_0 (H - M) $$
Magnetic Moments
-
magnetic materials always act as dipoles (even as a small piece of magnetic material, carries double magnetic charge - N and S poles)
-
therefore, possible to define a
magnetic dipole moment
-
each atom of magnetic material acts as a magnetic dipole
-
electric orbits acts like a coil generating magnetic field.
-
the material can be considered as an assembly of blocks - current loops
-
the magnitude of magnetic dipole μ
m
= Ai
-
A = area of current loop
-
i = value of the current in the loop
-
in macroscopic body, net current in interior blocks is 0 because neighbouring currents cancel each other
-
on the surface, there is non-zero net current which equals iAn, where n is the number of blocks
-
every atoms except noble gases should demonstrate magnetic properties (inequal number of +- m
l
and +- m
s
)
-
this is valid for gases only
-
all magnetic compound materials include at least one transition element
-
these elements have an
inner
incomplete electron shell
-
as we learned, when atoms bond they electrons fill their *
outer
electron shell
-
only materials with incomplete inner shells after bonding
show magnetic properties
Size of Atomic Magnetic Moment
-
when atomic sub-shells are being filled, the m
l
are first filled with electrons have m
s
= +1/2 and only then electrons having m
s
= -1/2 start to occupy their states
-
the consequence of this is that electrons with two different functions and the same spin have a lower energy then electrons with different wave functions and different spins
Bohr Magneton
-
β = qh/4πm - fundamental unit of magnetic moment
Alignment of Magnetic Moments in a Solid
-
rules for magnetic solids:
-
closed shells give no magnetic moment
-
this is no μ
orb
for 3d shells
-
third effect is the interaction between the electron spins of adjacent atoms
-
this interaction can vary in strength. When it is strongest, it caries magnetic moments of adjacent atoms to either be
parallel
or
anti-parallel
to each other
Paramagnetics
-
spin interactions are negligible compared to thermal agitation of the atomic magnetic moments
-
directions of magnetic moments in atoms are randomly oriented
-
an external magnetic field can induce some degree of alignment but it disappears when field is removed
Ferro-, Antiferro- and Ferrimagnetism
Ferromagnetics
-
strong spin interactions cause the atomic magnetic moments to align parallel to each other
-
in these metals, 3d electrons from the incomplete shells can move around the materials
-
the ability of electrons to move reduces the interaction ebtween the electrons and hence the magnetic moments are also reduced
Antiferromagnetics
-
strong spin interactions lead to these atomic magnetic moments to be anti-parallel to each other in adjacent atoms
-
in the whole material, the number of atoms with anti-parallel magnetic moments are equal
-
net magnetic moment in the material is zero
Ferrimagnetics
-
the moments for adjacent atoms are in opposite directions HOWEVER the moments have different magnitudes, thus giving us a non-zero net magnetic moment
-
this feature stems from the nature of these materials: they are all compounds
-
ferrimagnetics are electrical insulators, unlike all useful ferromagnets
-
ferrimagnetics can be used for high-frequency applications to avoid high eddy currents and resultant losses
-
ferrimagnetic compounds are called
ferrites
Diamagnetics
-
in diamagnetics, intrinsic magnetic field is opposite to external field therefore it is expelled from the field
-
all materials are diamagnetic, but the effect is weak and only shows up when none of the other effects are present
-
diamagnetism arises due to Lenz’ Law: “when a magnetic is moved towards a loop of wire, it induces a current in the loop which in turn generates a magnetic field to oppose the magnetic’s motion”
Curie Temperature
-
beyond a certain temperature, a material ceases to be ferromagnetic at all, and M drops to 0
-
at T = 0K, all atomic moments are perfectly aligned as we assume in our calculation. At T > 0K, thermal excitation of atoms reduces the degree of alignment and M drops
-
the temperature at which M -> 0 is known as the
Curie temperature
Domains and Hysteresis
-
if all the atomic moments are lined up, this represents the maximum value of the moment - the condition known as
saturation
-
why are ferromagnetic elements not always permanent magnets?
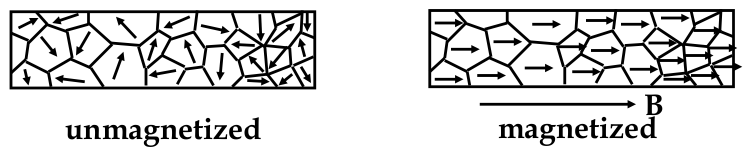
-
materials are divided into sub-units known as
domains
-
each domain is magnetized to saturation (inside domain, all moments are aligned)
-
however, direction of each domain can be randomly oriented
The B-H Curve
-
since M relates B and H and M is not constant, what is the relationship between B and H in practice?
-
H = external field, B = magentic flux density inside material
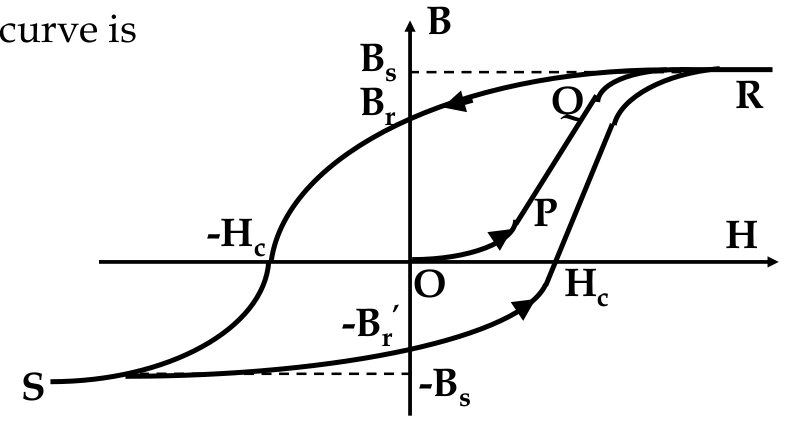
- as H increases, B follows the path O P Q R
- at R, B value saturates at B
s
- B
s
corresponds to complete alignment of atomic moments
- if the applied field is now reduced
after
increasing to R, the path follows a different direction
- at H =0, B = B
r
, known as remanence
- material now has a “permanent” magnetic flux density, becoming a permanent magnet
- to turn B to zero, apply a reverse field
- H
c
= coercive field or coercivity
- if we further increase B in the reverse direction, get saturation at -B
s
again
-
hysteresis:
the different behaviour of the B-H curve for different directions of H, and this curve is called the
hysteresis loop
Hard and Soft Materials
-
magnetic materials are divided into two broad groups based on their B-H curves:
magnetically hard
and
magnetically soft
materials
-
this refers to how easy it is to magnetize or demagnetize them
-
measure of magnetic hardness is the value of H
c
-
soft materials have a high permeability
Domains
-
domain’s formation is caused by energy minimization reasons
-
if all domains were aligned, then material would be magnetic and much of the field would be outside the material
-
but the field is a “storage” of potential energy called
magnetostatic energy
-
the whole energy in the system could be reduced by forming more domains
-
following this logic, the best result can be achieved if we have atomized domains
-
however, we have to consider the boundaries between domains, the
domain walls
-
domain wall:
the boundary between the two domains where the magnetization changes from one direction to another and hence the atomic spins do too
-
not simply one atomic spacing wide
-
exchange forces between neighbouring atomic spins favour very little relative rotation
-
magnetic moments that are oriented away from the field direction possess excess potential energy call the
anisotropy energy
-
in domain wall region, there is competition between exchange forces and the anisotropy energy minimization
-
what happens when we apply external magnetic field to the ferromagnet?
-
domains aligned parallel to the field will expand at the expense of domains with other orientations
-
growth is called the
motion of domain walls
-
the stronger the magnetic field, the more non-parallel domains shrink. As their size is small enough, the magnetic moments in these domains can be rotated until the material is uniformly magnetic
-
in magnetically soft materials, domain size shrinkage and magnetic moments rotation occur simultaneously and at low field
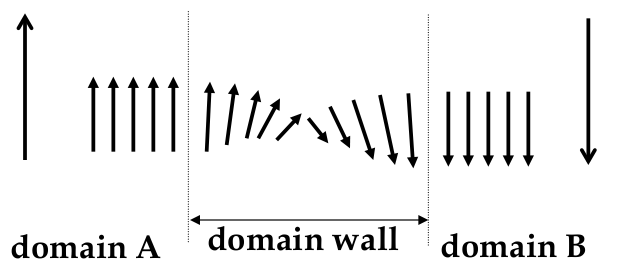
Domains in Polycrystals
-
in polycrystalline materials, grain boundaries do not allow domain walls to expand
-
after external field is applied, domain wall motion will result in crystal grains with single domains
-
in many grains, magnetic moments will be pointing at some angle to H
-
after we remove the field, some of these domains will return to previous conditions but some remain resulting in
remanence
B
r
on the B-H curve
-
heat above T
c
or strong reverse field -H use to reverse the effect
Magnetic Recording


![]()